Indian National Chemistry Olympiad Examination
1999
Maximum Points: 80
- Please wear your apron at all times while you are in the laboratory.
- Carefully read the text of the experiment.
- You have 4 hours to complete the experimental task, and record your result
on the answer sheet provided to you. You must stop your work immediately when
the examination time is over.
- Do not leave the examination room until you are directed to do so.
- Use distilled water, chemicals, and glassware with care.
- Chemicals or glassware can be asked for, if used up or broken.
- Show at least one endpoint of all the titrations to the examiner and take
the examiner's signature on the constant readings before proceeding for the
calculations.
- This question paper has 4 pages.
- Any accident should be reported to the examiner immediately.
Introduction:
You will be estimating lead and tin content of the given sample by
complexometric titration, a technique similar to the neutralisation or
oxidation-reduction titrations. The titration which will be performed in this
experiment is based upon a particular class of co-ordination compound called as
chelates. A chelate is produced when a metal ion coordinates with two or more
donor group of a single ligand. Depending upon the number of donor groups
available for coordination bonding, chelating reagent is named as bi-, ter-,
quadri-, quinque- or hexadentate. In this experiment, you will be using a
Ethylenediaminetetraacetic acid (EDTA), a most widely used complexometric
hexadentate ligand for titration.
Theory:
EDTA has following structure-
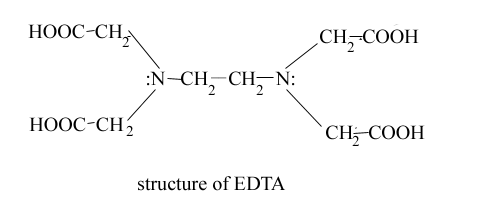
EDTA is a polyaminocarboxylic
acid. In addition to four acidic hydrogen, each nitrogen has one unshared pair
of electrons. The molecule thus has six potential sites for bonding with metal
ion. Valuable property of EDTA as a titrant is that it combines with metal ions
in 1:1 ratio regardless of the charge of the cation, and thus gives a sharp
endpoint. EDTA in its disodium form is generally used for complexometric
titrations which is abbreviated as Na2H2Y.2H2O.
Both Pb2+ and Sn4+ will react with EDTA as follows-
Pb2+ + H2Y-2 ----> PbY-2 +
2H+
Sn4+ + H2Y-2 ----> SnY + 2H+
In the current experiment, a mixture of Sn4+ and Pb2+
ions is complexed by addition of excess EDTA solution. Excess EDTA is then back
titrated with standard lead nitrate solution using xylenol orange as an
indicator. At the endpoint, slight excess of Pb2+ forms a red complex
with xylenol orange and thus the colour of the solution changes from yellow to
red. This titration gives the amount of EDTA which is consumed by the total Pb
and Sn content of the sample. While conducting the titration, 30% hexamine
solution is added to maintain the pH of the sample solution around 6.
When a
fixed amount of sodium fluoride is added to the same sample solution, the
fluoride ions form a stable complex with Sn4+, but does not react
with Pb2+ at pH 6. The reaction of tin with fluoride ions can be
represented as:
SnY + nF- + 2H+ ---->
SnFn(n-4)- + H2Y2-
where n varies
from 4-6.
Thus, in presence of sodium fluoride, EDTA from Sn-EDTA complex is liberated
which can be titrated once again with standard lead nitrate solution. EDTA
released destroys a small amount of Pb-xylenol orange complex present in the
solution which is formed at the endpoint of the earlier titration. Thus, free
xylenol orange (yellow in colour) is liberated in the solution which acts as an
indicator.
To estimate lead and tin content in the given sample of alloy
Dissolution of supplied sample
You are given a weighed quantity of an alloy. Carefully transfer the sample in a
250 mL beaker. Add 10 mL of conc. HCl & 2-3 mL of conc. nitric acid. Keep
the solution at room temperature for 10-15 mins. with intermittent stirring.
Then, gently heat the beaker on hot plate (around 40-500C) until the
sample dissolves. If burner is used then gently heat the solution using a low
flame (if required, discontinue heating after some time). At this stage, the
solution is generally dark brown in colour and also has some brown colour spongy
mass either floating or adhering to the beaker. The spongy mass is a flux
material which will not dissolve in the acids added. Do not try to dissolve or
filter the flux material as it does not interfere with the analysis. View the
beaker from bottom against light. Presence of any grey particles indicate that
your sample is not yet completely dissolved. To ensure complete dissolution of
the sample, gently heat the solution further. You may have to add the stated
quantities of acids twice or thrice, if the sample does not dissolve. After the
sample is totally dissolved, boil the solution to expel the nitric acid from the
solution (about 2 to 3 mins). At this stage, the colour of the solution is
generally pale yellow. Avoid total drying of the solution. Cool the solution
slightly. A white precipitate will appear in the solution.
Concentrated acids are very corrosive. The steps above involve hot
solutions. Be careful of vapours!
Preparation of sample solution
To the above solution, add 25 mL of 0.2M EDTA solution supplied to you. Stir
well and boil the solution for 1 or 2 minute in order to dissolve the
precipitate formed. Dilute the solution with approximately 100 mL of distilled
water. Cool the solution for 5 mins and then transfer it to 250 mL standard
volumetric flask. Dilute the solution upto the mark with distilled water. This
is your sample solution.
Titration with Lead Nitrate
- Fill the burette with 0.01 M Pb(NO3)2
- Quickly, pipette out 25 mL of the sample solution to a 250 mL conical
flask. To this flask, add 15 mL of hexamine solution and 100 mL of distilled
water and 5 to 10 drops of xylenol orange indicator. The colour of the
solution will be yellow. Simultaneously, prepare three flasks in this manner(#
1, 2, & 3).
- Titrate the solution from flask (#1) with standard
Pb(NO3)2 solution until the colour of the solution
changes from yellow to orangish red (or red). Note down the reading.
- To the same flask(#1), add 2g of solid sodium fluoride provided to you.
The solution will turn yellow in colour.
- Titrate the solution with Pb(NO3)2 till the colour
changes from yellow to orangish red (or red). Note down the reading.
- Perform titrations for flask #2 and #3 in similar manner.
Blank Titration
- Pipette out 25 mL of 0.2M EDTA supplied to you in a 250 mL standard
volumetric flask. Dilute the solution upto the mark with distilled water.
- Pipette out 25 mL of diluted EDTA solution in a 250 mL conical flask, add
1 mL conc HCl, 15 mL hexamine solution and 100 mL distilled water.
- Titrate the solution with standard Pb(NO3)2 using
xylenol orange indicator, until the colour changes from yellow to red. Note
down this reading. Take at least 3 readings.
|
Calculate the percentages of Lead and Tin in the sample provided to
you. Present your readings for all titrations in appropriate form on the
answer sheet provided. Show all the necessary calculations.
|
Atomic masses: Pb 207.2, Sn 118.7
